Constraining the air-sea exchange of inorganic and methylated mercury with high resolution spatial and temporal measurements in the Sargasso Sea (NSF Chemical Oceanography)
The project will evaluate the concentrations of Hg in the surface ocean and atmosphere in the waters around Bermuda to examine the factors controlling their concentration and chemistry, and the magnitude of the Hg sources and sinks. There is limited data examining the seasonal and temporal variability of Hg speciation in the surface ocean at a specific location, and its air-sea exchange, and this is the study’s primary focus. Measurements will be made in the North Atlantic Ocean near Bermuda, during both monthly and on six specific Hg-focused cruises. Atmospheric samples will be collected as well at the atmospheric sampling station in Bermuda. During cruises, high resolution Hg speciation data in the atmosphere (gaseous and aerosol) and surface waters (gaseous elemental Hg, dissolved and particulate ionic Hg, MeHg and dimethylmercury) will be gathered to examine the sources of Hg and its transformations, and how this impacts the formation of MeHg. Additionally, measurements of Hg stable isotopes in atmospheric and water samples will be used to identify sources (e.g., geogenic or anthropogenic inputs) and delivery mechanisms of Hg to this region. This proposed research will further assess the importance of natural variability, human activity, and climate change on altering Hg and MeHg levels in the ocean, and in marine organisms consumed by humans.
The Effect of Terrestrial Organic Matter on Coastal Mercury Cycling (NSF Chemical Oceanography)
The project is collaborative research with colleagues at Dartmouth College (Vivien Taylor and Celia Chen). The study was designed to examine the effects of climate-driven increases in terrestrial organic matter (OM) concentrations in the Gulf of Maine (GoM) waters on the complex and often competing processes influencing: 1) mercury (Hg) cycling and distribution; 2) the formation of methylated Hg (both monomethyl (MeHg) and dimethylmercury (DMeHg)); and 3) MeHg uptake into microplankton. Our central hypothesis is that higher terrestrial OM inputs, and likely hightened co-transport of Hg and MeHg, and concomitant shifts in coastal OM amount and quality will cause increases in Hg inputs, MeHg production and influence its bioaccumulation. Terrestrial OM is thought to be a conduit for transfer of Hg from watersheds to coastal and offshore waters and controls the formation of aqueous MeHg by providing a microenvironment that supports microbial Hg methylation. Increasing dissolved OM concentrations have been associated with decreased bioaccumulation of MeHg into phytoplankton, the primary bioaccumulation step for uptake of MeHg into seafood. However, our preliminary results show this association is mediated by OM source more than concentration, as indicated by the sulfur content of the OM. Furthermore, mixotrophic microplankton could bioaccumulate MeHg complexed with OM while feeding heterotrophically. Mixotrophs can seasonally dominate microplankton assemblages in coastal waters, and the impact of heterotrophic feeding modes on MeHg uptake at the base of the food web has not been adequately explored. The proposed research aimed at studying the effects of OM dynamics on Hg and MeHg cycling and bioaccumulation through 1) field surveys and shipboard experiments in the GoM, where significant increases in terrestrially-sourced OM have caused a “yellowing” effect, and 2) through laboratory microcosm experiments using autotrophic and mixotrophic microplankton taxa under contrasting carbon acquisition modes and OM characteristics and concentrations. The field program is built around 5 proposed cruises in the GoM and the laboratory studies will include both uniculture uptake experiments and mesocosm studies.
The first cruise was completed in April 2023 – after being delayed from Nov 2022 – and Rob, Hannah, Sophia and Melissa were on board. We collected water samples throughout the water column at 15 stations in the GoM and in the Penobscot River, which is historically contaminated with Hg from industrial sources. Dissolved and particulate samples were collected for total Hg and MeHg, and on-board analysis was completed for dissolved gaseous Hg species, both Hg0 and DMeHg. Additionally, large particulate samples were collected using McLane pumps and plankton nets for the analysis of Hg natural isotope abundance. an underway Hg0 sampler also collected samples continuously during the cruise. Size fractionated seston samples were also collected. Colleagues from Bigelow Laboratory were also measuring OM characteristics and other ancillary parameters on the cruise.
Prior to the cruise, phytoplankton uptake experiments with a mixotrophic dinoflagellate were done examining the pathways of MeHg accumulation. Further experiments are planned.
US GEOTRACES GP-17- OCE and -ANT Sections: External Sources, Cycling and Processes Affecting Mercury Speciation in the South Pacific and Southern Oceans (NSF Chemical Oceanography)
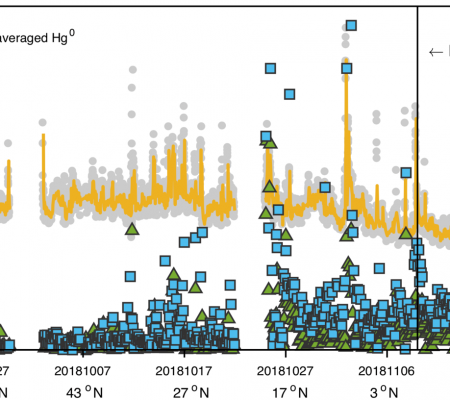
The major goals of the project were to participate in two oceanographic cruises that form part of the GEOTRACES GP17 activities (GP17-OCE in 2022/23 and GP17-ANT in 2023/24), and do ancillary sampling and analysis at the institutions covered by this collaborative award to ensure the success of the project. The overall goals of the collaborative study were based on the priorities of the GEOTRACES program and associated activities, and the gaps in knowledge concerning mercury (Hg) ocean biogeochemistry. To achieve the aims of the research, measurements are being made of all the Hg species: total Hg (HgT), elemental Hg (Hg0), methylmercury (MMHg) and dimethylmercury (DMHg), in the dissolved and particulate phases, as appropriate, and in the atmosphere. The UConn and USGS studies focused on collecting samples for examining the air-sea exchange of Hg and methylated Hg, and the isotopic composition of these fractions. The other collaborators are UC-Santa Cruz and Wright State University. The collaborative group proposed a program with the major objectives being focused by the following hypotheses: 1) Atmospheric deposition is the primary external Hg source and the major factor controlling the evasion rate of Hg0 to the atmosphere in the South Pacific and Southern Oceans; 2) While continental, deeper ocean margins and hydrothermal inputs are not important Hg sources, these inputs dominate external fluxes of MMHg; 3) MMHg formed in ice and at the sediment-water interface is an important source to the Southern Ocean; 4) The fraction of total Hg as methylated Hg is related to primary production and associated deeper water remineralization such that the South Pacific gyre will have some of the lowest relative concentrations in the global ocean; 5) Localized external inputs of Hg, such as glacial melt, are not exceptionally high (unlike in Greenland), but the flux associated with increased freshwater inputs is observable. Likewise, fluxes of Hg into the ocean from sediment porewaters will be low, but locally important on the Antarctic shelf; and 6) Dissolved total Hg isotopic composition in surface water will elucidate the relative contributions of dry and wet deposition, as well as glacial ice melt, across a gradient of climatic conditions from the Southern Pacific to the Southern Ocean. Hypotheses 1-4 are relevant to both cruises while hypotheses 5 & 6 relarted primarily to GP17-ANT.
The first cruise (GP17-OCE) has been completed at the end of the current funded year and preliminary results and data suggest that the cruise was a success in terms of achieving the major objectives. Preliminary underway data is shown above. preparations for the second cruise are already beginning.
Constraining the Role of Chemical Transformations in the Cycling of Mercury at the Arctic Ocean Air-Sea Interface (NSF Polar Programs)
The project is focused on examining the air-sea exchange of mercury (Hg) forms, both inorganic (ionic (HgII) and elemental (Hg0) and organic forms (monomethyl (MeHg) and dimethyl Hg (DMeHg)), in the Arctic Ocean and the role of sea ice in mediating the gas exchange of Hg0 and methylated Hg, and how ice cover influences the extent of the chemical reactions in surface waters and Hg gas exchange. Additionally, given the region of the study, the interaction between the sediment and water column, and the distribution of Hg species throughgout the water column were also examined. The study has provided information on how climate change and other factors are affecting the levels of MeHg in the water, and thus the marine food chain, and how this could impact human exposure now and in the future. To enhance understanding, radon (Rn), an inert gas, was also be measured by our collaborators to allow for the separation of the role of gas exchange versus the role of chemical reactions for the Hg forms. Other radioisotopes were also measured to enhance understanding of other aspects of the Hg cycle. The hypotheses driving this research were primarily examined during a cruise in the Arctic Ocean using field measurements, which occurred in May/June 2021, and through on-going laboratory experiments during the cruise, and experiments at the University of Connecticut (UConn). The hypotheses are: 1) Gas evasion of elemental Hg0 is not the primary sink for water column Hg in the Arctic Ocean; 2) The relatively low net production of Hg0 in the Arctic reflects lower UV light levels and ice cover, low productivity and low bioavailable HgII for reduction; and 3) Gas evasion of DMeHg is not the primary sink for methylated Hg and in situ decomposition is the major loss mechanism in Arctic seawater.
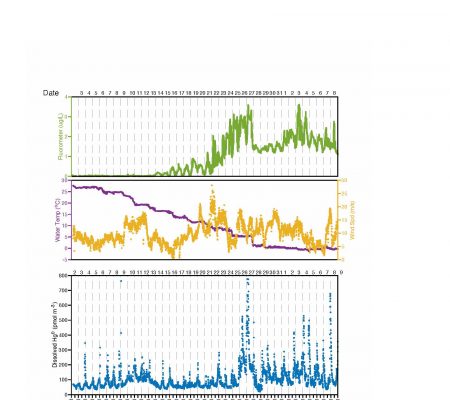
To examine these hypotheses in detail, the following objectives have been completed: 1) During a late spring cruise in the Arctic Ocean, along a transect from open water, through the marginal ice zone and into completely covered ice regions, high resolution measurements of dissolved Hg0 and atmospheric Hg speciation (Hg0, reactive gaseous Hg (RGHg) and aerosol Hg (PHg)), as well as the concentrations of Hg species in the water column and sediments, and in sea ice were made. Determination of surface water DMeHg also occurred; 2) Samples were collected to assess the Rn deficit with respect to its parent, radium, in surface waters for the estimation of gas exchange rates and how this deficit changes with ice cover; 3) Underway ancillary data on water temperature, salinity, fluorescence, air temperature, UV, and PAR, wind speed and direction, were collected as needed for flux estimation and data interpretation, as well as ancillary samples for the measurement of Hg speciation (Hg0 , MeHg, DMeHg) and related parameters in surface waters, surface snow and sea ice; 4) Atmospheric precipitation and aerosol samples were collected during the cruise; 5) On-board incubation experiments, and further pre- and post-cruise studies, have been completed to examine the photochemical transformations, and the microbially-mediated (dark) redox processes influencing inorganic Hg and methylated Hg speciation, and the controlling factors; and 6) this information is being used to produce a revised budget for inorganic Hg and methylated Hg cycling within the mixed layer of the Arctic Ocean using our data and results from the literature.
This project is currently on a no-cost extension and will end at the end of 2023. The following publications are related to this project.
He, Yipeng and Shi, Xiangming and Huffman, Wesley W. and Lamborg, Carl H. and Mason, Robert P.. (2022). Description of a Dimethylmercury Automatic Analyzer for the High-Resolution Measurement of Dissolved Gaseous Mercury Species in Surface Ocean Waters. Environmental Science & Technology. 56 (18) 13076 to 13084.
Dastoor, Ashu and Angot, Hélène and Bieser, Johannes and Christensen, Jesper H. and Douglas, Thomas A. and Heimbürger-Boavida, Lars-Eric and Jiskra, Martin and Mason, Robert P. and McLagan, David S. and Obrist, Daniel and Outridge, Peter M. and Petrova, Mariia V. and Ryjkov, Andrei and St. Pierre, Kyra A. and Schartup, Amina T. and Soerensen, Anne L. and Toyota, Kenjiro and Travnikov, Oleg and Wilson, Simon J. and Zdanowicz, Christian. (2022). Arctic mercury cycling. Nature Reviews Earth & Environment. 3 (4) 270 to 286.
Marissa C. Despins, Robert P. Mason, Ana M. Aguilar-Islas, Chad R. Hammerschmidt, Silvia E. Newell. Linked mercury methylation and nitrification across oxic sub-polar regions. Submitted to Frontiers in Environmental Chemistry, in revision .
Nine presentations at meetings have been made at national and international conferences on the mercury studies. The project supported the thesis work of Yipeng He and Marissa Despins and will form part of the thesis research of Hannah Inman. The direct collaborators on the project were Dave Kadko (deceased), Mark Stephens (FIU) and Doug Hammond (USC). Other researchers on, or receiving data from the cruise, included Penny Vlahos and students (UConn), and Laurie Juranek (Oregon Univ.) and Bob Pickart (WHOI)