Completed Projects
Methylated Mercury Sources and Cycling in the High Latitude North Atlantic (NSF Chemical Oceanography)
This project, which was completed in June 2023, was designed to addresses key questions regarding the benthic mercury (Hg) contribution in the ocean and its potential impact on our understanding of the biogeochemical cycling of Hg species, both inorganic and organic Hg, their inputs to the deep ocean, and the sources and cycling of methylated Hg (TMeHg) in the subpolar North Atlantic. The research was focused by a GEOTRACES Process Cruise around Iceland on which we participated in summer 2021. Our research was formulated around the hypotheses that: 1) Benthic sediments are a net source of TMeHg to the deep water in this region and will dominate the TMeHg supply into the North Atlantic; 2) While sediment may release inorganic Hg due to various biochemical and physical processes, sediments will be a net sink for THg; and 3) Overall, the seas around Iceland are a net sink for total Hg but a source for TMeHg to the deep North Atlantic Ocean, with the sources being primarily from shelf- and sediment-derived TMeHg inputs. The hypotheses wee trsted by making radioisotopic and Hg measurements on the cruise and analysis of this data in conjunction with the known hydrography and water flow patterns in this region of the ocean.
The GEOTRACES expedition (64PE474, July-August 2021) allowed for various natural radiotracer approaches to be used to quantify the various exchange fluxes, and while the post-doc on the cruise was involved in these on-board activities, she also collected samples of dissolved and particulate THg and TMeHg, and also processed samples for sediment and porewater Hg species. The radiotracer methods used allowed for better estimation of benthic Hg fluxes than has been done previously. Analysis of data to date show that the benthic fluxes of dissolved MeHg and total Hg can be quantified with the 224Ra/228Th disequilibrium approach. The rate of particulate Hg redeposition was also traced with the excess 234Th of the top sediment layer. Results indicate that the sediment on the sides of Greenland-Iceland Rise was a main source of Hg species to this region. The strong underwater current of the Denmark Strait likely carries these Hg inputs southwards into the North Atlantic. For the sampling stations on the top of the rise, excess 224Ra and excess 234Th relative to their parents were observed in the sediment column, suggesting the redeposition of the resuspended particles. The concentrations of MeHg and total Hg in the bottom water at stations at the top if the rise were lower than those at the rise side stations, implying that the redeposited particles scavenged considerable Hg from water column. Sediments on the rise are likely a sink for Hg transported from the Arctic but a source for MeHg. Overall, the results support all the proposed hypotheses about the role of sediments as a methylated Hg source but a sink for inorganic Hg. The initial results were presented at the international mercury meeting in July 2022, and at other meetings. A paper is in preparation. Final analysis of samples is ongoing, as is data synthesis, quality assurance and data analysis.
NSF Environmental Chemistry: Examining the role of nanoparticles in the formation and degradation of methylated mercury in the ocean
This study was a collaboration with Jing Zhao in the Chemistry Department began in summer 2016 and was funded through the Environmental Chemistry program at NSF. Research examined the role of cadmium selenide (CdSe) and other nanoparticles (NPs) in the transformations of methylmercury (MeHg). Published results are Shi et al. (2021) which examined the transformations of inorganic and MeHg in the presence of cysteine-capped CdSe NPs, and built on from Wang et al., 2019, who examined the interactions of inorganic Hg and MeHg with various types of NPs, using various techniques. See here. The overall project is focused on understanding how environmental surfaces, and in particular, nanoparticles influence the formation and degradation of methylated Hg species (MeHg and dimethylmercury (DMeHg)). The idea is built on our initial studies with iron sulfide minerals (FeS) and thiols which have shown that these can mediate the conversion of MeHg into DMeHg through a methyl transfer reaction (Jonsson et al., 2016). Formation and/or precipitation of HgS is the other product of this reaction.
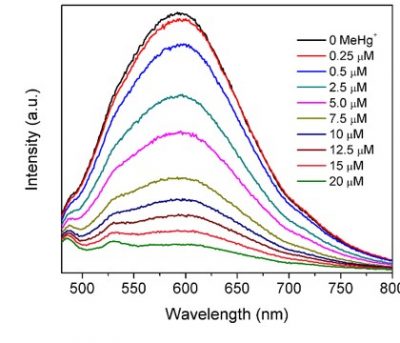
The overall motivation for these studies is that while DMeHg is found throughout the ocean water column there is little understanding of its formation. Additionally, while MeHg formation appears to be microbially mediated, it is not known whether DMeHg is formed by biotic or abiotic processes. We hypothesized that nanoparticles (NPs) would mediate reactions in the marine environment that are important pathways for the abiotic formation of DMeHg and the decomposition of MeHg. We have examined the interactions of inorganic Hg and MeHg with cadmium sulfide (CdS) and selenide (CdSe) and found an interaction which is manifest in a decrease in the fluorescence of the NP solutions – see figure. Further study of the interactions using XPS and ICP-MS has shown that the interaction is not between the added Hg and the NP core, and there is no replacement of Cd with Hg, but that the interaction is only at the NP surface. These findings have important implications for understanding the role of natural and manufactured NPs in the environment on the bioavailability and transformation of MeHg. Given the interactions found, it is less likely that formation of DMeHg will occur in the presence of NPs, but this is being further studied. Current results suggest that at lower pH, the surface of the NPs becomes more active, perhaps due to the dissociation of the capping agent and we have evidence that a reaction occurs, leading to a decrease in the MeHg concentration. We have not yet ascertianed the exact nature of the product, which is volatile, as we do not obtain a mass balance in solution after acidifying. We propose to further examine this as a potential abiotic formation of DMeHg. Further studies will focus on using natural and manufactured nanoparticles (FeS, CdS, CdSe and HgS), coated with different organic compounds, and with DOC, and how the form and surface characteristics may impact the rate of reaction, and what type of thiols may be important in these reactions under environmentally-relevant conditions. We also examine other potential abiotic degradation pathways for MeHg that do not lead to DMeHg formation.
Studies focused on the interaction if inorganic Hg and methylmercury (CH3Hg) with nano-particles (NPS) and also metal sulfide microparticles. The studies with the microparticles focused on the potential for ionic Hg (Hg(II)) to be reduced to elemental Hg (Hg(0)). This was demonstrated and the specific conditions that enhanced this transformation were established in the presence of iron sulfide (FeS) and the extent of the reaction depended on pH and other factors. Additionally, the presence of natural orgnaic matter in the solution altered the reaction extent. The thermodynamics of the various interactions were examined and detailed. In contrast to FeS, there was no reaction in the presence of CdS particles. These studies were detailed in Coulibaly et al. (2021).
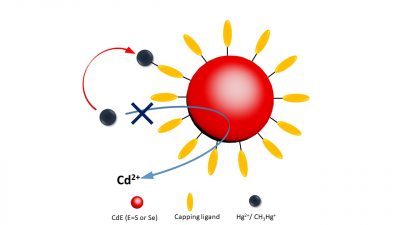 In contrast to the studies with microparticles, it was shown that Hg(II) interacted strongly with the NPs, in this case CdSe NPs, but that there was no formation of Hg(0). However, the Hg(II) became strongly associated with the NPs and quenched their fluorescence substantially. The degree of quenching interaction was a function of the added Hg(II) concentration. The interaction is represented in the associated figure. Addition of CH3Hg resulted in a more muted response and it is suspected that the CH3Hg had to be degraded to Hg(II) for a significant interaction to occur. Light enhanced this degradation, likely by enabling the dissociation of the surface ligand, or its degradation, and exposing the bulk NP to the CH3Hg, promoting its reaction. In the presence of NPs, no (CH3)2Hg was formed which is counter to the results found with micro FeS particles by Jonsson et al. (2016).
In contrast to the studies with microparticles, it was shown that Hg(II) interacted strongly with the NPs, in this case CdSe NPs, but that there was no formation of Hg(0). However, the Hg(II) became strongly associated with the NPs and quenched their fluorescence substantially. The degree of quenching interaction was a function of the added Hg(II) concentration. The interaction is represented in the associated figure. Addition of CH3Hg resulted in a more muted response and it is suspected that the CH3Hg had to be degraded to Hg(II) for a significant interaction to occur. Light enhanced this degradation, likely by enabling the dissociation of the surface ligand, or its degradation, and exposing the bulk NP to the CH3Hg, promoting its reaction. In the presence of NPs, no (CH3)2Hg was formed which is counter to the results found with micro FeS particles by Jonsson et al. (2016).
Pathways for the formation of (CH3)2Hg were examined as part of the study and it was found not to occur in the presence of the NPs. However, there is the potential for this to occur artifactually in the CH3Hg standards at their high concentrations, and that future experiments should take this possibility into account. While (CH3)2Hg was not formed in the presence of NPs and CH3Hg, it was clear however that the CH3Hg was degraded and the Hg(II) formed as a result was co-precipitated onto the NP surface. This conclusion was supported by measurements of Hg and Cd in each fraction. Clearly, this was not an exchange reaction where Hg replaces Cd, but a surface interaction with the NP. The results of these experiments are contained in Wang et al. (2019) and Shi et al. (submitted).
Mercury in the Hackensack
Funding from the Hudson River Foundation supported the project “Evaluation of the impacts of human and climate disturbance on mercury dynamics and bioaccumulation in benthic and pelagic organisms in the Hackensack sub-estuarine system”. The study focused on the Hackensack sub-estuary of the Hudson River system. The research involved fieldwork and samples were analyzed and data is still being compiled but the results show interesting trends. A presentation was made at the International Conference on Mercury as a Global Pollutant (ICMGP) in Kraow, Poland in 2019. Laboratory mesocosm experiments were also completed suring the the project, using an annular flume that was used to generate sediment resuspension for the studies examining the impacts of sediment transport on mercury (Hg) and methylmercury (MeHg) in different marsh environments. The primary focus was examining the role of sediment resuspension and transport in the fate and mobility of total Hg and MeHg within the system and the potential for re-contamination of areas, that have been remediated, from external local sources, and the role of vegetation in mediating net Hg methylation. The main objectives of the project were: 1) to determine Hg and MeHg contamination levels at three sites within the Hackensack River, including one at the mouth of Berry’s Creek, to ascertain whether Berry’s Creek could serve as a source of the observed recontamination up and downstream from its convergence with the Hackensack River; 2) investigate the patterns of MeHg concentrations in the Hackensack River ecosystem, by assessing total and MeHg in various benthic and pelagic organisms found at sampling sites, along with ancillary variables that will help identify sources; 3) examine how the water flow conditions, i.e. regular tidal current vs. storm-induced river flow, can influence the mobility of inorganic Hg and MeHg from sediment into the water column; 4) assess the effectiveness of Spartina vs. Phragmites marshlands in trapping contaminated particulate transported from other reaches by the river, and in the production of MeHg from inorganic Hg inputs; and 5) employ phytoplankton uptake experiments to determine key factors driving Hg and MeHg bioavailability to phytoplankton, the entry point to aquatic food webs in the system. The research was recently highlighted y the university’s Office of Research. Papers are being prepared for publication.
Superfund Program: Methylmercury Production and Fate in Response to Multiple Environmental Factors
This project, a sub-project of a multi-investigator project with Dartmouth College “Sources and protracted effects of early life exposure to arsenic and mercury”, was funded through the NIH Superfund Program. The project used experimental approaches, field studies, and modeling to investigate the combined and interactive effects of environmental changes in temperature, salinity, and organic carbon concentration (OC) on the fate of MeHg in marine ecosystems. The collaborative nature of the project was that most of the geochemical analyses and related studies are completed at UConn while the focus of the biological analysis and bioaccumulation studies were done at Dartmouth, within Celia Chen’s research group. Plankton bioaccumulation studies were being completed in Nick Fisher’s lab at Stony Brook as another aspect. For more details on the overall project click here.
More details and results are detailed below. Two papers have been published related to this work – Seelen et al. (2021) in Water Research and Buckman et al., (2021) in Environmental Research. The research, which builds on previous work completed under the Superfund program, was focused on examining the factors controlling MeHg production, in both the water column and sediments, and the net input from sediments to the water column, and how these influence bioaccumulation in coastal food webs, and ultimately impact human exposure to MeHg. Interactions between environmental factors were evaluated by adapting various approaches to distinguish additive from non-additive effects. Experimentally parameterized, field validated Hg methylation and demethylation assays, bioaccumulation and exposure models were developed to predict the impact of complex environmental alterations on MeHg fate in marine ecosystems. The major hypotheses of the project were: 1) MeHg production, and flux from sediments to the water column, will decrease due to the combined and interactive effects of increasing carbon loading to sediments, temperature, and salinity on MeHg production and mobility; 2) In estuarine field sites spanning a range of comparable OC, salinities, and Hg inputs, Hg methylation rates and %MeHg in sediments from southern latitudes will be higher than those from more northern latitudes; 3) Bioaccumulation of MeHg in phytoplankton will decrease due to combined and interactive effects of increasing dissolved OC, temperature and salinity on MeHg bioavailability, phytoplankton growth and metabolism; 4) The trophic transfer of MeHg from primary producers through primary to secondary consumers will increase due to combined and interactive effects of the major variables – the cumulative impact of the changes expected due to increasing OC, temperature, and salinity will decrease MeHg production but increase bioaccumulation in the food web; and 5) The cumulative impact of the changes expected due to increasing OC, temperature, and salinity will result in a decrease in human exposure to MeHg through coastal seafood consumption.
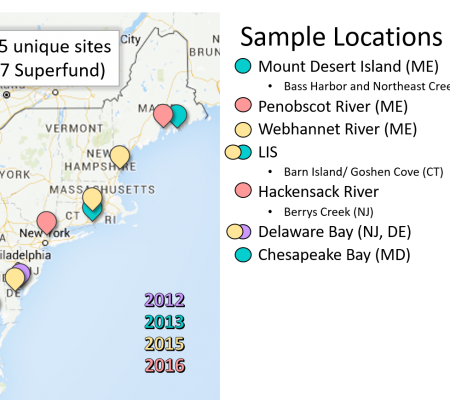
Other studies include a detailed mesocosm study, which has been published (Buckman et al., 2019; see publications) which examined the interactions between sediment organic content and temperature on the bioaccumulation of MeHg into amphipods, oysters and plankton, and examination of the differences in the extent of MeHg production and flux from the sediment to the water column. Field studies within estuaries along the east coast of the US (Delaware to Maine) in 2015 and 2016 were aimed at better understanding the links between sediment and water column characteristics and sediment MeHg production, and flux (both particulate and dissolved), and how the complexation of Hg within the dissolved and particulate phase impacts its methylation. Results from this work are published (Taylor et al., 2019; Seelen et al., 2018). These studies build on our previous work within the region (Balcom et al., 2015; Gosnell et al., 2016; Chen et al., 2014). Studies were also focused on better understanding the bioavailability and MeHg content of different water column particulate fractions as our previous work has shown that forage fish MeHg burdens correlate better with water column concentrations than with those of bulk surface sediment (Chen et al., 2014). We were investigating this link in greater detail in recent studies. Additionally, in 2016, studies were focused on examining Superfund (contaminated sites) locations to examine how Hg and MeHg cycling may differ in these ecosystems compared to less impacted locations. A number of papers have been published, and presentations related to this work have been made at national and international meetings, including the ICMGP in Krakow in Sept 2019..
One study (Seelen et al., 2018), looked at examining the composition of the resuspended material compared to that of the bulk surface se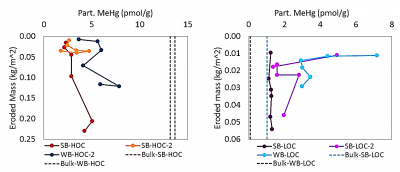 diment (0-4 cm). Sediments collected from the Delaware River from high organic (HOC) and low organic matter (LOC) locations from two sites (SB and WB) were used for sediment resuspension experiments using Gust erosion devices. The collected resuspended sediment was analyzed for Hg, MeHg and ancillary information including C and N stable isotopes. Overall, the resuspended sediment did not closely match the bulk sediment (see figure. bulk meHg indicated by dotted lines) and better reflected the composition of the resuspended particulate. This study further suggests that bulk sediment characteristics are not reflective of what is entering the base of the food chain in estuaries. Another study, completed by Sofi Jonsson, a research scientist from Sweden, and Nash Mazrui, a PhD student, in 2015 examined how complexation of Hg to different dissolved ligands and particulate fractions impacted the rate of Hg methylation. Results suggest that Hg bound to DOC is more bioavailable that Hg bound to other ligands, and that Hg bound to iron sulfide is the least bioavailable. A paper related to this work has been submitted for publication. Results from one experiment are shown in the figure. The methylation rate was higher in the LOC sediments compared to the HOC sediments in all cases. Furthermore, the Hg pre-equilibrated to DOC (HgII-DOM in the figure) was higher than that of an inorganic Hg solution spike (HgII(aq)) and also when the aqueous spike and DOC were added simultaneously but not pre-equilibrated (HgII(aq)+DOM in the figure).
diment (0-4 cm). Sediments collected from the Delaware River from high organic (HOC) and low organic matter (LOC) locations from two sites (SB and WB) were used for sediment resuspension experiments using Gust erosion devices. The collected resuspended sediment was analyzed for Hg, MeHg and ancillary information including C and N stable isotopes. Overall, the resuspended sediment did not closely match the bulk sediment (see figure. bulk meHg indicated by dotted lines) and better reflected the composition of the resuspended particulate. This study further suggests that bulk sediment characteristics are not reflective of what is entering the base of the food chain in estuaries. Another study, completed by Sofi Jonsson, a research scientist from Sweden, and Nash Mazrui, a PhD student, in 2015 examined how complexation of Hg to different dissolved ligands and particulate fractions impacted the rate of Hg methylation. Results suggest that Hg bound to DOC is more bioavailable that Hg bound to other ligands, and that Hg bound to iron sulfide is the least bioavailable. A paper related to this work has been submitted for publication. Results from one experiment are shown in the figure. The methylation rate was higher in the LOC sediments compared to the HOC sediments in all cases. Furthermore, the Hg pre-equilibrated to DOC (HgII-DOM in the figure) was higher than that of an inorganic Hg solution spike (HgII(aq)) and also when the aqueous spike and DOC were added simultaneously but not pre-equilibrated (HgII(aq)+DOM in the figure).
US GEOTRACES Pacific Meridional Transect: Determination of the Air-Sea Exchange of Inorganic and Methylated Mercury in the Anthropogenically-Impacted and Remote Pacific Ocean
The research, funded by the NSF Chemical Oceanography Program, was aimed at addressing key parameters outlined in GEOTRACES documents, and, in particular, is: 1) helping to assess the anthropogenic impact of Hg from the atmosphere to the Pacific Ocean, while informing more generally about the sources of Hg and methylmercury (MeHg) in the sampled air, precipitation and aerosols (natural vs anthropogenic); 2) quantifying the evasion of Hg species (elemental (Hg(0)) and dimethylmercury (DMeHg)) and the distribution of Hg and MeHg in the atmosphere and in wet deposition, which will help assess the variation in deposition during the cruise (GP15 in figure), and constrain the estimation of the net input of Hg to the surface ocean; and 3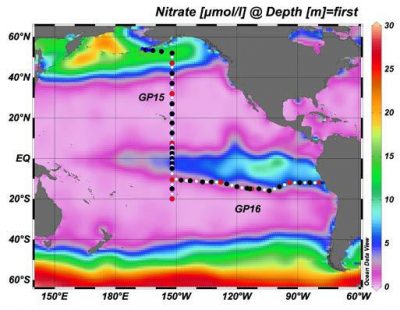 ) quantifying the air-sea exchange of methylated Hg (both MeHg and DMeHg) which will lead to a better understanding of the importance of deposition as a source of mixed layer MeHg. The research was designed to test the following hypotheses concerning the air-sea exchange of Hg, and the sources and sinks for ocean Hg: 1) atmospheric Hg deposition is the primary factor controlling the evasional flux of Hg(0) to the atmosphere in the North and tropical Pacific Ocean; 2) evasion of DMeHg is a small component of the gas exchange flux but its degradation in the atmospheric boundary layer is an important source of atmospheric MeHg; 3) scavenging of gas and aerosols is the main source for the MeHg found in wet deposition; and 4) halogen chemistry plays a substantial role in Hg cycling within the marine atmospheric boundary layer and elevated ionic Hg concentrations will be found in regions with low ozone. Samples were collected during the 2018 GEOTRACES cruise in the atmosphere continuously using a Tekran speciation unit as well as with ion exchange membranes at lower resolution for reactive gaseous Hg (RGHg) and particulate Hg, and aerosols were also collected using hi-volume samples, along with wet deposition. A paper comparing the measurements made with the Tekran and with the CEM membranes has recently been published in Atmospheric Environment.
) quantifying the air-sea exchange of methylated Hg (both MeHg and DMeHg) which will lead to a better understanding of the importance of deposition as a source of mixed layer MeHg. The research was designed to test the following hypotheses concerning the air-sea exchange of Hg, and the sources and sinks for ocean Hg: 1) atmospheric Hg deposition is the primary factor controlling the evasional flux of Hg(0) to the atmosphere in the North and tropical Pacific Ocean; 2) evasion of DMeHg is a small component of the gas exchange flux but its degradation in the atmospheric boundary layer is an important source of atmospheric MeHg; 3) scavenging of gas and aerosols is the main source for the MeHg found in wet deposition; and 4) halogen chemistry plays a substantial role in Hg cycling within the marine atmospheric boundary layer and elevated ionic Hg concentrations will be found in regions with low ozone. Samples were collected during the 2018 GEOTRACES cruise in the atmosphere continuously using a Tekran speciation unit as well as with ion exchange membranes at lower resolution for reactive gaseous Hg (RGHg) and particulate Hg, and aerosols were also collected using hi-volume samples, along with wet deposition. A paper comparing the measurements made with the Tekran and with the CEM membranes has recently been published in Atmospheric Environment.
Aerosol and deposition samples have been analyzed for total Hg and MeHg. Continuous dissolved gaseous Hg was sampled underway, and also surface samples collected to determine methylated Hg. Water column profile samples of total MeHg were also collected. The measurements made relied on well-developed, routinely used techniques but also built on new approaches to examine important questions concerning the cycling of methylated Hg across the ocean-air interface. Method development prior to the cruise ensured that the collections at sea, both underway measurements and samples collected for later analysis at the University of Connecticut, will yield significant and detailed results. Sample analysis is currently underway and the continuous measurements are still being processed, although most atmospheric samples have been analyzed. Preliminary results f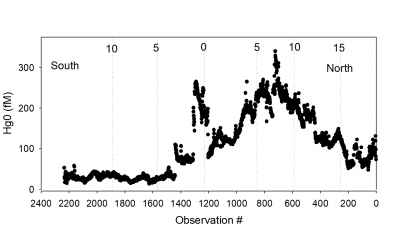 or dissolved gaseous Hg are shown in the figure at left. More detailed analysis of these data are underway. Presentations of the results will be made at the ICMGP in Krakow, Poland in Sept 2019, and at the Chemical Oceanography Gordon Research Conference in July 2019. The results from the research will provide data required by the GEOTRACES program and enhance our understanding of the cycling of Hg and methylated Hg species at the sea-air interface. This information is vital to understanding the long-term consequences of anthropogenic Hg releases on MeHg levels in seafood, and associated impacts on human and wildlife health, and also inform the Minamata Convention. A PhD student, and undergraduate students, are being educated and gaining research experience during the project, which will be a focus of the graduate student’s thesis.
or dissolved gaseous Hg are shown in the figure at left. More detailed analysis of these data are underway. Presentations of the results will be made at the ICMGP in Krakow, Poland in Sept 2019, and at the Chemical Oceanography Gordon Research Conference in July 2019. The results from the research will provide data required by the GEOTRACES program and enhance our understanding of the cycling of Hg and methylated Hg species at the sea-air interface. This information is vital to understanding the long-term consequences of anthropogenic Hg releases on MeHg levels in seafood, and associated impacts on human and wildlife health, and also inform the Minamata Convention. A PhD student, and undergraduate students, are being educated and gaining research experience during the project, which will be a focus of the graduate student’s thesis.
Collaborative Research: Transformations and Mercury Isotopic Fractionation of Methylmercury by Marine Phytoplankton
This project, which was funded by NSF Chemical Oceanography is a collaboration with Nick Fisher at Stony Brook more and John Reinfelder at Rutgers more and began in fall 2016. The major goals of the collaborative research project were focused on addressing the following hypotheses: 1) Reduction of ionic Hg (HgII) within algal cells and algal formation of dimethylmercury (Me2Hg) and/or elemental Hg (Hg0) from methylmercury (MeHg) are important pathways for the formation of volatile Hg species in the surface ocean, and coccolithophores and possibly other haptophytes account for production of most of the volatile Hg; 2) Production of Me2Hg occurs through reactions of MeHg with thiols and Se-proteins within cells, and through its abiotic interaction with particulate or nanoparticulate sulfides within the water column; and 3) Transformations of Hg species within phytoplankton impart distinct Hg isotopic signatures on residual Hg in phytoplankton that are unique for HgII and MeHg and different from those of abiotic photochemical reactions. To test these hypotheses, our groups examined the transformations and isotopic fractionation of Hg by marine phytoplankton in laboratory and field experiments. The studies at UConn were focused mostly on abiotic transformations, although field studies collected seston samples for analysis, and also examined methylation in coastal waters.
Additionally, studies were focused on understanding more clearly the factors influencing the uptake of MeHg into phytoplankton. Preliminary results are shown below for a study that looked at the uptake of MeHg in the presence of two marine DOC samples extracted from coastal waters (Orrington and Sawyer Island), and compared the rates to published data in the literature. As shown in the figure on the left, the recent study results are consistent with Luengen et al. (2012) that the uptake rate decreases with DOC concentration, but with substantial scatter in the data. 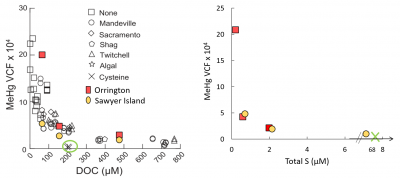 Also, uptake in the presence of cysteine was much lower than with DOC in the previous study. When the recent data and the cysteine data are plotted against the total reduced S content of the DOC (right figure), the relationships is more consistent, reinforcing the expectation that it is not the total DOC content but rather the reduced S content that determines the extent of binding of MeHg to the DOC, and therefore the impact on bioaccumulation. More studies were done to examine how the character of the DOC influences uptake and transformations of MeHg.
Also, uptake in the presence of cysteine was much lower than with DOC in the previous study. When the recent data and the cysteine data are plotted against the total reduced S content of the DOC (right figure), the relationships is more consistent, reinforcing the expectation that it is not the total DOC content but rather the reduced S content that determines the extent of binding of MeHg to the DOC, and therefore the impact on bioaccumulation. More studies were done to examine how the character of the DOC influences uptake and transformations of MeHg.
The overall studies examined further the factors controlling uptake and the the extent of biologically mediated and abiotic production of volatile Hg species, and the transformation of MeHg into other Hg forms. While prior studies of the Mason group have focused on the photochemical formation of Hg0 and the degradation of MeHg, the current studies were more focused on field measurements of bioaccumulation and the relationship to size and water column parameters, and seasonal changes, as well as the transformations in the presence of microbes.
Influence of Nitrogen on Methylmercury Accumulation in Shellfish and Planktivorous Fish Along the Long Island Sound Shoreline of Connecticut
This collaboration with Zosia Baumann was funded by Connecticut Sea Grant (NOAA) and began in early 2016 (2/2016- 2018). We began sampling locations along the CT coastline in spring 2016 and there were further collections in 2017. Samples are collected for sediment, water and biota. The hypotheses that were tested with the project are: 1) methylmercury (MeHg) concentrations in sediments and water will be lowest at sites with the highest levels of total nitrogen (N); and 2) High total N concentrations will be associated with an overall decrease in MeHg concentrations in phytoplankton cells, bivalves, and fish. These two hypotheses reflected our overall initial assessment of the complex interactions between nutrient and carbon levels and MeHg production and bioaccumulation. The overall objectives were to examine links between nutrients, including dissolved and particulate species of N and phosphorus (P) and the net Hg methylation rate and the influence of N on Hg and MeHg uptake and consequently bioaccumulation in marine phytoplankton, bivalves and fish. We expected that while more nutrients could stimulate more methylation in sediments, overall the various interactions, such as the effects of nutrients and carbon on MeHg bioavailability and organism 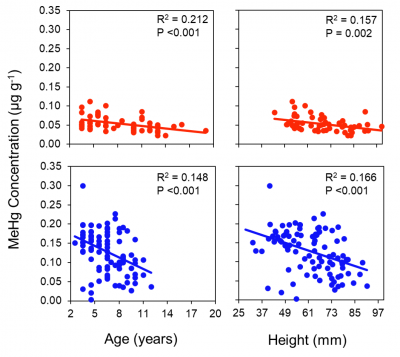 growth, will lead to less bioaccumulation at the more eutrophic locations. Gunnar Hansen is the PhD student working on the project and Wes Huffman collected zooplankton as a side project to examine concentrations in these organisms in more detail. One interesting aspect of the study was a collaboration with Robert Cerrato at Stony Brook in aging the clams collected.
growth, will lead to less bioaccumulation at the more eutrophic locations. Gunnar Hansen is the PhD student working on the project and Wes Huffman collected zooplankton as a side project to examine concentrations in these organisms in more detail. One interesting aspect of the study was a collaboration with Robert Cerrato at Stony Brook in aging the clams collected.
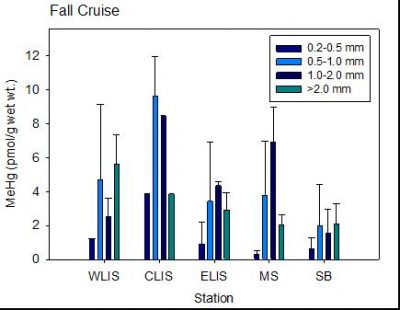
We found that in contrast to fish, MeHg in clams does not increase with age – see figure. The results of the study have been presented at various conferences and papers from this work are being prepared for publication. Results for the concentrations of MeHg in silversides collected in various coastal embayments (Newark, Stratford, Jordan Cove and Mumford Cove), including various sites in New Haven Harbor, are shown in the attached figure. Concentrations do not correlate strongly with 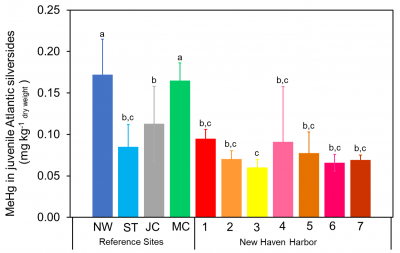 sediment Hg concentrations, suggesting the role of other factors in influencing MeHg bioaccumulation.
sediment Hg concentrations, suggesting the role of other factors in influencing MeHg bioaccumulation.
Atmospheric Measurements and Air-Sea Exchange on the US Arctic Geotraces Cruise
A paper published in 2018 (DiMento et al., 2018) highlighted many of the results from our involvement in the Arctic GEOTRACES cruise, a collaborative project with colleagues at University of Tennessee Space Institute (Steve Brooks) and Chris Moore, funded by the NSF Chemical Oceanography program. Additionally, a Scientia article highlighted some of the activities related to the air-sea exchange of mercury in the Arctic and in other locations, focusing on NSF funded studies and those of Swedish collaborators. The Arctic is being substantially altered by climate change and this will have a significant impact on the biogeochemistry of Hg in the region and its bioaccumulation as methylmercury (MeHg) into Arctic marine seafood and marine mammals. The major activity of the NSF grant was the participation in the Artic GEOTRACES research expedition on the research vessel Healy, which occurred from August to November, 2015. The cruise proceeded from Dutch Harbor to the North Pole and back. The UConn research group was responsible the underway sampling equipment for measurement of dissolved gaseous elemental Hg (Hg(0)). The equipment was maintained by Strve Brooks on board during the cruise, and others helped in collection of samples of ice cores, snow and mlt ponds samples that were shipped and analyzed at UConn. The UConn group was responsible for the analysis of atmospheric mercury samples (wet deposition, gas spec iation and aerosols) collected on board. Overall, the impact of ice and other factors on the magnitude of the air-sea exchange fluxes is hotly debated but poorly characterized. The underway dissolved gaseous mercury analyzer collected high resolution samples of Hg(0) in conjunction with high resolution air collections (see figure for the water data) that was used to estimate gas-exchange, and examine factors influencing Hg0 concentration and distribution. Overall, while much higher concentrations of Hg(0) were found under the ice, with low values in the ice-free zones, the atmospheric concentration was very consistent throughout the cruise. The differences are much more pronounced than expected based on other studies in the Arctic and elsewhere. These activities and related analyses will allow for a clearer understanding of the magnitude of atmospheric deposition to the Arctic Ocean, and gas evasion to the atmosphere. The results formed part of Brian DiMento’s PhD thesis – he graduated in May 2017.
iation and aerosols) collected on board. Overall, the impact of ice and other factors on the magnitude of the air-sea exchange fluxes is hotly debated but poorly characterized. The underway dissolved gaseous mercury analyzer collected high resolution samples of Hg(0) in conjunction with high resolution air collections (see figure for the water data) that was used to estimate gas-exchange, and examine factors influencing Hg0 concentration and distribution. Overall, while much higher concentrations of Hg(0) were found under the ice, with low values in the ice-free zones, the atmospheric concentration was very consistent throughout the cruise. The differences are much more pronounced than expected based on other studies in the Arctic and elsewhere. These activities and related analyses will allow for a clearer understanding of the magnitude of atmospheric deposition to the Arctic Ocean, and gas evasion to the atmosphere. The results formed part of Brian DiMento’s PhD thesis – he graduated in May 2017.
Methylmercury Interactions with Marine Plankton
This project, w hich finished in 2/2016, was funded through NSF Chemical Oceanography, and was collaboration with the research groups of Nick Fisher at Stony Brook, who focused on examining the impacts of water chemistry and other factors on phytoplankton uptake and trophic transfer, and Elsie Sunderland at Harvard, whose group focused on the modeling. Kati Gosnell, the student who worked on the project, graduated in spring 2016. The overarching objective of the field, lab and modeling studies was to provide insight into the mechanism of entry of methylmercury (MeHg) into marine food webs and how ocean properties
hich finished in 2/2016, was funded through NSF Chemical Oceanography, and was collaboration with the research groups of Nick Fisher at Stony Brook, who focused on examining the impacts of water chemistry and other factors on phytoplankton uptake and trophic transfer, and Elsie Sunderland at Harvard, whose group focused on the modeling. Kati Gosnell, the student who worked on the project, graduated in spring 2016. The overarching objective of the field, lab and modeling studies was to provide insight into the mechanism of entry of methylmercury (MeHg) into marine food webs and how ocean properties
influence its production, uptake and subsequent bioaccumulation. The research focused around three main objectives: 1) Characterize the variability in uptake of MeHg and Hg by diverse marine phytoplankton and trophic transfer to zooplankton as a function of biological attributes and relevant environmental and physical variables; 2) Examine the relative importance of decomposing phytoplankton and fecal pellets as a source of MeHg to marine waters compared to production during carbon degradation by bacteria; and 3) Refine the global oceanic budget for MeHg including the role of primary and secondary producers in MeHg production, transport and accumulation based on lab and field data. The research at UConn mostly focused on the field component, but also measured the variability in MeHg uptake into marine phytoplankton and its trop-hic transfer to zooplankton under controlled lab conditions. Studies in the field looked at the impact of biological differences (cell walls, size) and environmental factors and composition of DOC and specific organic ligands. The studies analyzed correlations between Hg and MeHg measured in size fractionated plankton and over different vertical depths from various ocean cruises and coastal/shelf sites and compared these to ancillary data on water chemistry. Two papers have been produced to date: Gosnell and Mason (2015) reported on samples collected in the tropical Pacific Ocean; Gosnell et al. (2016) was focused on studies within Long Island Sound a
nd adjacent waters
(see attached figures). Both phytoplankton and zooplankton size classes were analyzed for their Hg and MeHg concentrations. In addition, in the LIS study, stable isotopes analyses of C, N and S in plankton were used to further assess sources of MeHg to plankton and the differences in sources for different parts of LIS and offshore waters. Data was also incorporated into Schartup et al. (2015), which focused on the sources of MeHg in Lake Melville.
Overall, the study assisted in identifying the vector for MeHg entry and accumulation in marine food-webs, which is a critical gap in our present knowledge. As shown in the attached figures, the concentrations of MeHg in zooplankton were comparable for the open ocean and coastal waters even though the water MeHg concentrations were higher in the coastal waters. These differences were even more enhanced for the phytoplankton.
Photochemical Redox Chemistry of Mercury and Selenium and the Degradation and Transformations of Their Methylated Compounds
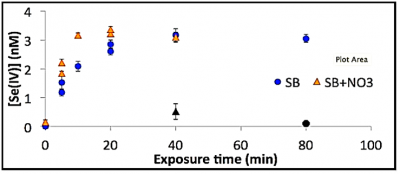 This work was initially funded by a NSF grant but even though the grant has been completed the work continued as the results provided new insights into the air-sea exchange of mercury (Hg) and selenium (Se). The work was completed by Brian DiMento, a PhD student who graduated in May 2017, with model development of a global Se model being done by Rob, in collaboration with Harvard researchers. The results of the photochemical studies are applicable to other studies, such as GEOTRACES, and to the study of the global and ocean cycling of Se. Studies examining the photochemical oxidation of selenite and the reduction of selenite showed that these reactions are very slow under environmental conditions. However, in contrast, methylated Se compounds are rapidly degraded. Results from one experiment examining the decomposition of dimethylselenium in coastal shelf break (SB) seawater is shown in the attached figured. The product of the decomposition, selenite (Se(IV)), is monitored in these experiments and shows the rapid degradation of the methylated compound in the presence of light. Various experiments suggest that the rate constant for degradation is about 45 per day (up to 10-3 per sec). There was some indication that nitrate enhanced the reaction rate. In the dark, the compound is stable under the time constraints of the experiment. The photochemical results, data from the Metzyme cruise and the selenium global model were published in 2018 in Global Biogeochemcial Cycles (Mason et al.).
This work was initially funded by a NSF grant but even though the grant has been completed the work continued as the results provided new insights into the air-sea exchange of mercury (Hg) and selenium (Se). The work was completed by Brian DiMento, a PhD student who graduated in May 2017, with model development of a global Se model being done by Rob, in collaboration with Harvard researchers. The results of the photochemical studies are applicable to other studies, such as GEOTRACES, and to the study of the global and ocean cycling of Se. Studies examining the photochemical oxidation of selenite and the reduction of selenite showed that these reactions are very slow under environmental conditions. However, in contrast, methylated Se compounds are rapidly degraded. Results from one experiment examining the decomposition of dimethylselenium in coastal shelf break (SB) seawater is shown in the attached figured. The product of the decomposition, selenite (Se(IV)), is monitored in these experiments and shows the rapid degradation of the methylated compound in the presence of light. Various experiments suggest that the rate constant for degradation is about 45 per day (up to 10-3 per sec). There was some indication that nitrate enhanced the reaction rate. In the dark, the compound is stable under the time constraints of the experiment. The photochemical results, data from the Metzyme cruise and the selenium global model were published in 2018 in Global Biogeochemcial Cycles (Mason et al.).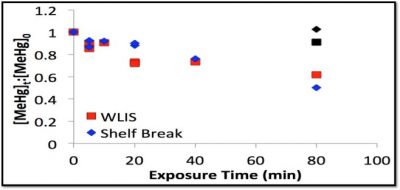
The rate of degradation is much faster for methylated Se than that of MeHg under similar conditions. Again, MeHg is stable in the dark. In comparable experiments, with western Long Island Sound (WLIS) and SB water, shown in the figure below, the rate of degradation is about an order of magnitude slower. There was no significant difference in the rate for the different waters. This has generally been found to be so for a variety of coastal waters. Differences in the DOC content or the salinity do not appear to have a large impact on the photochemical demethylation rate.
Interestingly, while the concentrations of MeHg are much higher in coastal waters and marshes, and the degradation rates at the surface are similar, because of differences in light attentuation, the water column integrated degradation rate is higher for open ocean waters than for coastal waters, and this is also enhanced because of the much higher UV penetration in the open ocean. the results of these studies are compiled in a paper in Marine Chemistry (DiMento et al.).
Climate Impact on Coastal Ecosystem Methylmercury: Human Exposure Implications
This project, which finished in summer 2016, was funded through the NSF/NIH Oceans and Human Health Initiative and was a collaboration between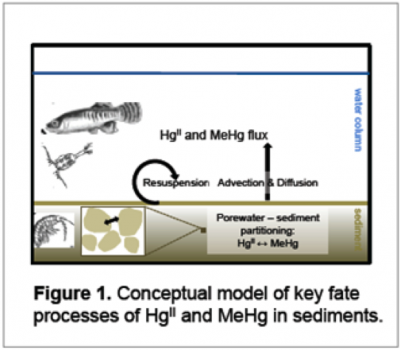 Rob Mason and Evan Ward at UConn and Celia Chen and Brian Jackson at Dartmouth College. The goals of the proposed research were to use experimental approaches, field studies, and modeling to investigate the interactive effects of changes in temperature and nutrient loading/carbon content associated with climate on the fate of MeHg in marine ecosystems. The research focused on MeHg production, input to the water column, and bioaccumulation in coastal food webs, and its potential impact on human exposure to MeHg. The specific aims of the project were to: 1) Determine the effects of temperature, nutrient and carbon loading on the net MeHg formation in sediments and the associated net inputs of MeHg to the water column in coastal ecosystems; 2) Determine th
Rob Mason and Evan Ward at UConn and Celia Chen and Brian Jackson at Dartmouth College. The goals of the proposed research were to use experimental approaches, field studies, and modeling to investigate the interactive effects of changes in temperature and nutrient loading/carbon content associated with climate on the fate of MeHg in marine ecosystems. The research focused on MeHg production, input to the water column, and bioaccumulation in coastal food webs, and its potential impact on human exposure to MeHg. The specific aims of the project were to: 1) Determine the effects of temperature, nutrient and carbon loading on the net MeHg formation in sediments and the associated net inputs of MeHg to the water column in coastal ecosystems; 2) Determine th
e interactive effects of temperature and nutrient supply/ecosystem eutrophication on bioaccumulation of MeHg by primary producers, and transfer between primary and secondary consumers in estuarine food webs; and 3) Predict how climate-induced changes in MeHg bioaccumulation in coastal food webs will increase exposure and risk to sensitive populations. The attached conceptual model was used to focus the studies completed under this project. The Dartmouth collaborators mostly focused on the bioaccumulation and biotic studies while the UConn group focused on understanding the geochemical
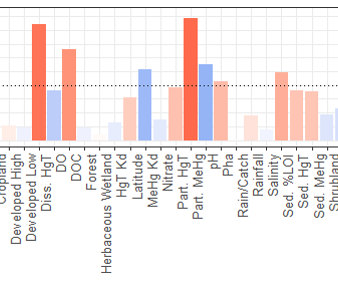
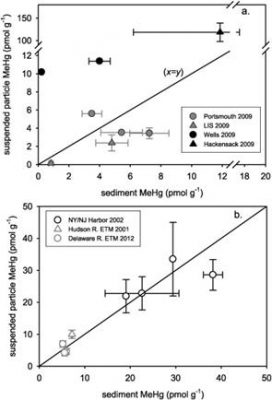
interactions in sediments and the water column and the exchanged between the different reservoirs. The field and laboratory studies (various micro and mesocosm experiments) tested our hypotheses on how temperature and carbon/nutrients impacted net methylation in sediments, its transfer to the water column, and bioaccumulation in the estuarine food webs. These approaches yielded a matrix of data that is being used to parameterize a Hg ecosystem and exposure model. To date, a number of publications have resulted from this work, which have highlighted the major factors influencing bioaccumulation in estuarine ecosystems: Balcom et al. (2015) examined correlations between different fractions and differences across ecosystems as shown, for example in the attached figure. The correlations for MeHg differ for different ecosystems. The figure illustrates that while in some estuaries, such as the Hudson and Delaware Rivers, there is a reasonable correlation between suspended particulate and surface sediment MeHg, this is often not the case, as found in Long Island Sound, Portsmouth Harbor and other locations. These results are consistent with what was reported in Chen et al. (2014) where MeHg concentrations in forage fish did not correlate with sediment MeHg levels but did correlated with suspended particulate MeHg, indicating the importance of the base of the pelagic food chain in transferring MeHg even to forage fish that feed at the sediment interface.
Examining the Biogeochemical Cycling of Mercury and Methylmercury in Lake Melville, Labrador, Canada
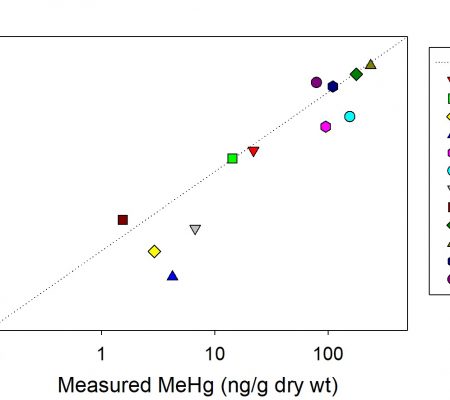
This project was completed in collaboration with Elsie Sunderland from Harvard University, at the request of the Nunatsiavut government. Harvard did most of the sample analysis and modeling and Amina Schartup, a former student, was the post-doc overseeing the proje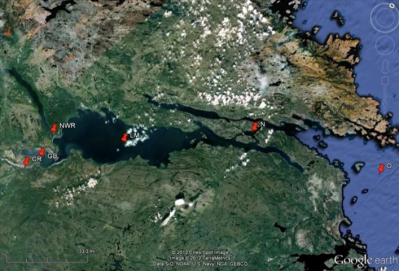 ct and doing most of the work . UConn personnel were involved mainly in the collection of water, sediment and biota samples on Lake Melville, a large saline water body in Labrador, Canada, and in the analysis of plankton samples. While called a “lake” it is actually connected to the ocean via a convoluted passage (see map). Much of the sample analysis was done at UConn. Results from this study were reported in Schartup et al. (2015) (see attached figure which presents a mass balance for the ecosyste
ct and doing most of the work . UConn personnel were involved mainly in the collection of water, sediment and biota samples on Lake Melville, a large saline water body in Labrador, Canada, and in the analysis of plankton samples. While called a “lake” it is actually connected to the ocean via a convoluted passage (see map). Much of the sample analysis was done at UConn. Results from this study were reported in Schartup et al. (2015) (see attached figure which presents a mass balance for the ecosyste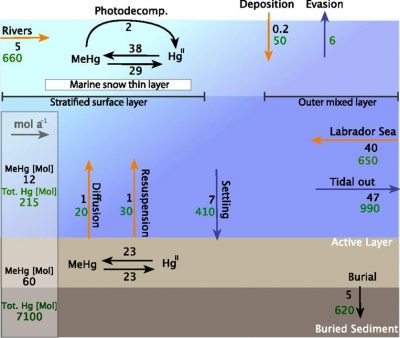 ms for methylmercury (MeHg)). Overall, the study examined the importance of water column methylation versus other sources of MeHg to the ecosystem, and confirmed its importance in this ecosystem. Furthermore, the study illustrated the potential impact of building a reservoir on the Columbia River, which has been proposed, on the future levels of MeHg in the ecosystem, which would likely increase as a result of the reservoir construction.
ms for methylmercury (MeHg)). Overall, the study examined the importance of water column methylation versus other sources of MeHg to the ecosystem, and confirmed its importance in this ecosystem. Furthermore, the study illustrated the potential impact of building a reservoir on the Columbia River, which has been proposed, on the future levels of MeHg in the ecosystem, which would likely increase as a result of the reservoir construction.
Figure: Mass balance for total mercury (red) and methylmercury (black) for lake Melville illustrating the importance of water column net methylation as a source relative to other inputs. Taken from Schartup et al. (2015); and used with permission.
Mercury Cycling in the Delaware River
This two year study examined the distribution and fate of mercury (Hg) and methylmercury (MeHg) in the Delaware River estuary through a project funded by the Delaware Department of Natural Resources and Environmental Control and the Delaware River Commission. The Mason group made seasonal field trips to the estuarine section of the river and examined both water and sediments in late fall, spring and summer. The main aims of the project were to: 1) characterize the concentrations and distributions of Hg and MeHg in the water column, at the surface and at depth, in the turbidity maximum, and sub-tidal region of the Delaware estuary, and in the associated sediments; 2) examine the rates of interconversion of Hg and MeHg in the upper sediments (top 12 cm) at three locations and relate this to sediment characteristics; and 3) analyze surface water samples collected over a year at a subset of stations along a longer reach of the river. The information gained was compared to data from other east coast US estuaries and the importance of sources of Hg and MeHg to the river and the aquatic food chain were examined. The Hg and MeHg information was used to derive an initial conceptual model of the factors important in Hg and MeHg cycling, fate and bioaccumulation within this region of the Delaware, and lead to the publication of Gosnell et al. (2015). One outcome of the project was a comparison of the relative importance of sediment MeHg inputs versus external sources. The paper concluded that there were times
seasonal field trips to the estuarine section of the river and examined both water and sediments in late fall, spring and summer. The main aims of the project were to: 1) characterize the concentrations and distributions of Hg and MeHg in the water column, at the surface and at depth, in the turbidity maximum, and sub-tidal region of the Delaware estuary, and in the associated sediments; 2) examine the rates of interconversion of Hg and MeHg in the upper sediments (top 12 cm) at three locations and relate this to sediment characteristics; and 3) analyze surface water samples collected over a year at a subset of stations along a longer reach of the river. The information gained was compared to data from other east coast US estuaries and the importance of sources of Hg and MeHg to the river and the aquatic food chain were examined. The Hg and MeHg information was used to derive an initial conceptual model of the factors important in Hg and MeHg cycling, fate and bioaccumulation within this region of the Delaware, and lead to the publication of Gosnell et al. (2015). One outcome of the project was a comparison of the relative importance of sediment MeHg inputs versus external sources. The paper concluded that there were times 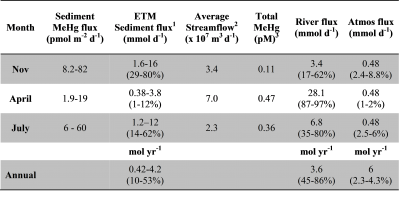 when sediment MeHg sources could be important, as summarized in the attached table.
when sediment MeHg sources could be important, as summarized in the attached table.
Table: Estimated fluxes of methylmercury to the water column at different times of the year, and overall. Data from Gosnell et al. (2015).
The Role of Marine Aggregates in Enhancing the Uptake of Nanomaterials and Mercury/Methylmercury by Bivalves
This work which has been completed over a number of years has been funded through Sea Grant and was done in collaboration with Evan Ward’s group, mostly by his students on the uptake mechanisms of various potential contaminants into bivalves, and the uptake of nanomaterials into bivalves. Two Sea Grant/NOAA projects were associated with this initiative: Emerging
contaminants in Long Island Sound: Effects of nanoparticles on suspension feeding bivalve molluscs (02/2012-02/2014) and Uptake and accumulation of nanoparticles of bivalves: implications of shellfish safety (02/2010-02/2013). Manufactured nanomaterials are used in a wide variety of ways and are released into the environment where they are also potentially toxic causing mitochondrial and DNA damage, and cell death. The study focus was how feeding of bivalve species affect their uptake of nanoparticles, and how formation of marine flocs enhances assimilation. Two papers were published as a result of this work: Doyle et al. (2015a) and (2015b). Link
Additionally, Roni Ortiz, a MS student who graduated in 2013, performed experiments examining the incorporation of mercury (Hg) and methylmercury (MeHg) into aggregates (“marine snow”) and how this accumulation may enhance the uptake of the Hg species into bivalves and their fate after ingestion. She completed an experiment using stable isotopes to track the incorporation, uptake and tissue redistribution of the mercury and methylmercury after feeding. One additional aspect of this study was that because the stable isotope spikes were used it was possible to examine the net transformation of inorganic Hg into MeHg during the experiments, and the converse, demethylation of MeHg. These was followed in the different size fractions and it was found that net methylation was enhanced in the larger particulate (aggregates), suggesting the formation of microzones where anaerobic bacteria, the principal methylators, were active due to oxygen depletion within the aggregates. The results were published in 2015 (Ortiz et al. 2015 ) and a figure showing the methylation and demethylation in the different size fractions is attached.
Examination of the Uptake of Mercury and Methylmercury using Bioreporters
This work was accomplished by Udonna Ndu while a PhD student within the department and also during his post-doctoral research at Rutgers University. The work used various “bioreporters” – bacteria that have been genetically modified so that the produce light in response to the uptake of mercury (Hg) and methylmercury (MeHg) compounds – and also through the examination of the production of elemental Hg. The focus was on the effects of large molecular weight or
ganic complexes as well as smaller thiols such as cysteine and glutathione. Results from these studies were published in a number of papers: a 2016 paper titled “The effect of aqueous speciation and cellular ligand binding on the biotransformation and bioavailability of methylmercury in mercury resistant bacteria” focused on Hg resistant bacteria while an earlier paper (The use of a mercury biosensor to evaluate the bioavailability of mercury-thiol complexes and mechanisms of mercury uptake by bacteria) focused on various aspects of the bioavailability of Hg and MeHg to E. coli and Bacillus strains. Finally, an earlier paper (set the groundwork for these more recent studies
Gas Exchange and Global Mercury Cycling
A long-term interaction with Elsie Sunderland at Harvard and her prior students/post-docs (Anne Soerensen, now at Stockholm University More) led to a number of studies related to air-sea exchange of elemental mercury and its role in the global Hg cycle. A paper by Amos et al. (2015) compared and contrasted model predictions to examine the impacts of legacy mercury, in particular early mining inputs prior to the industrial revolution. The ocean-atmosphere model (Soerensen et al., 2010) built on earlier models and papers, provided the basis for the examination of the factors controlling the flux of elemental Hg to the atmosphere. One of these studies was focused on the potential role of high concentrations of Hg from legacy
anthropogenic inputs on the flux (Soerensen et al., 2012).
A paper discussing the results from a cruise in the equatorial Pacific (Soerensen et al., 2014) highlighted the importance of atmospheric deposition in the high rainfall Inter-Tropical Convergence Zone in driving enhanced evasion in this region, and compared the results from this study with others in the Atlantic that found similar results. Modeling confirmed the importance of atmospheric deposition in driving the enhanced flux. This paper also provided a contrast to the studies completed in the North Atlantic where concentrations of elemental Hg, while overall higher, were much more consistent in concentration in the waters around Bermuda studied over multiple years (Soerensen et al., 2013).
Samples were also collected on a GEOTRACES cruise in the tropical South Pacific Ocean in 2014 in collaboration with Carl Lamborg and Chad Hammerschmidt’s research groups and others. The cruise was from
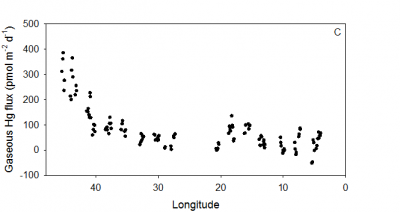
Ecuador to Tahiti, and underway samples were collected for dissolved gaseous Hg and atmospheric Hg, along with detailed water column sampling for mercury speciation (Bowman et al., paper in review). Estimated gas exchange rates are shown in the figure, which is part of a paper currently in preparation.
Atmospheric Deposition
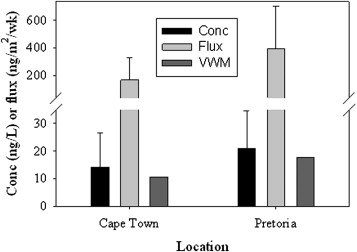
Studies were completed at a number of locations examining the wet deposition flux of Hg, and the importance of dry deposition of aerosols and of reactive ionic Hg (RGHg). Studies were completed in South Africa, and these were coordinated by the CSIR and the Weather Service, and an outgrowth of research completed in South Africa examining various aspects of mercury inputs and cycling. A paper examining deposition at Cape Point and in Pretoria was published in 2013. Another study was also completed on Bermuda which included measurements of atmospheric Hg and on aerosols (Gichuki et al., 2014). This study allowed for the examination of the importance of deposition in contributing Hg to this region of the ocean, and linked with the results of the Soerensen et al. (2013) study mentioned above.
Gulf of Mexico
A project completed with collaborators while they were at Harvard (David Senn, Bian Liu and others) examined the impact of hurricanes in the Gulf of Mexico and the role of bottom oxygen water depletion on the biogeochemistry of Hg and MeHg. Two papers were published from this work: a 2009 paper focused on the impact of the hurricanes while the 2016 paper was more focused on the potential impact of low oxygen conditions on Hg methylation. Methylation rates in the gulf of mexico were higher than previously reported for more temperate locations examined within the US and elsewhere.
Chesapeake Bay
A NSF-funded study focused on the distribution and formation of MeHg in the sediments of the Chesapeake Bay and the adjacent shelf and slope, examining the factors controlling the net production of MeHg in sediments and the flux of MeHg from sediments to the overlying waters. The project was a collaboration with Cindy Gilmour from the Smithsonian Environmental Research Center. more Two papers were published from this study: Hollweg et al. (2009) and (2010) – see publication list for details. The studies showed that the highest methylation rates were not in regions of the highest Hg concentration and provided further insights into the role of organic matter and sulfide in modulating both the rate of methylation and also the flux of MeHg from sediments. The examination of sediment fluxes took into account the role of speciation in modifying the overall flux.